- Mass Number Of Sulfur-34
- Mass Number Of Sulphur
- Mass Number Of Oxygen And Sulphur Atom
- Mass Number Of Sulphuric Acid
- Mass Number Of Sulphur Is 29
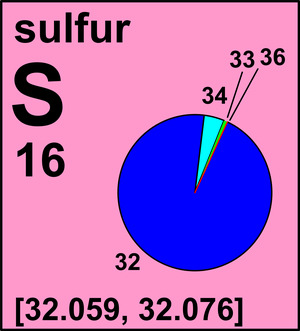
Number of electrons = 8. Mass number = Number of Protons + Number of neutrons. Hence mass number for Oxygen is 16. For Sulphur: Number of protons = 16. Number of electrons = 16. Mass number = Number of Protons + Number of neutrons. Sulfur (16 S) has 23 known isotopes with mass numbers ranging from 27 to 49, four of which are stable: 32 S (95.02%), 33 S (0.75%), 34 S (4.21%), and 36 S (0.02%). ›› Sulfur molecular weight. Molar mass of S = 32.065 g/mol. Convert grams Sulfur to moles or moles Sulfur to grams ›› Percent composition by element.
Molar mass of S = 32.065 g/mol
Convert grams Sulfur to moles or moles Sulfur to grams
| Symbol | # of Atoms | Sulfur | S | 32.065 | 1 | 100.000% |
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
Chemical properties of sulphur - Health effects of sulphur - Environmental effects of sulphur

|
SulphurSulphur is a multivalent non-metal, abundant, tasteless and and odorless. In its native form sulphur is a yellow crystalline solid. In nature it occurs as the pure element or as sulfide and sulfate minerals. Although sulphur is infamous for its smell, frequently compare to rotten eggs, that odor is actually characteristic of hydrogen sulphide (H2S). Applications The major derivative of sulphur is sulphuric acid (H2SO4), one of the most important elements used as an industrial raw material. Sulphur in the environment Life on Earth may have been possible because of sulphur. Conditions in the early seas were such that simple chemical reactions could have generate the range of amino acids that are the building blocks of life. Sulphur occurs naturally near volcanoes. Native sulphur occurs naturally as massive deposits in Texas and Louisiana in the USA. Many sulphide minerals are known: pyrite and marcaiste are iron sulphide ; stibnite is antimony sulphide; galena is lead sulphide; cinnabar is mercury sulphide and sphalerite is zinc sulphide. Other, more important, sulphide ores are chalcopyrite, bornite, penlandite, millerite and molybdenite. Health effects of sulphur
Effects of sulphur on the environment
Sources of periodic table. Back to the periodic table of elements. For more information on sulphur's place in the environment, move to the sulphur cycle. |
Mass Number Of Sulfur-34
More from 'Elements'
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
Mass Number Of Sulphur
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Mass Number Of Oxygen And Sulphur Atom
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Mass Number Of Sulphuric Acid
Mass Number Of Sulphur Is 29
Copyright © 1998-2021 Lenntech B.V. All rights reserved
