The negatively charged subatomic particle that moves around the nucleus is the electron. The proton, along with the neutron, comprises the nucleus. The only stable negatively charged subatomic particles is electron. All other hadronic (Pi-) and leptonic (muon-), negative charged particles are unstable. Well, antiprotons are negatively charged and are stable under condition they do not get in touch with matter hadronic particles. False, if you dont know the answer to that, its probably because you didnt know that a proton is a positively charged particle which forms a part of the nucleus. An electron is a negatively charged subatomic particle that moves around the nucleus.
Learning Objectives
- Describe the locations, charges, and masses of the three main subatomic particles.
- Determine the number of protons and electrons in an atom.
- Define atomic mass unit (amu).
Dalton's Atomic Theory explained a lot about matter, chemicals, and chemical reactions. Nevertheless, it was not entirely accurate, because contrary to what Dalton believed, atoms can, in fact, be broken apart into smaller subunits or subatomic particles. We have been talking about the electron in great detail, but there are two other particles of interest to us: protons and neutrons. We already learned that J. J. Thomson discovered a negatively charged particle, called the electron. Rutherford proposed that these electrons orbit a positive nucleus. In subsequent experiments, he found that there is a smaller positively charged particle in the nucleus, called a proton. There is also a third subatomic particle, known as a neutron.
Electrons
Electrons are one of three main types of particles that make up atoms. Unlike protons and neutrons, which consist of smaller, simpler particles, electrons are fundamental particles that do not consist of smaller particles. They are a type of fundamental particle called leptons. All leptons have an electric charge of (-1) or (0). Electrons are extremely small. The mass of an electron is only about 1/2000 the mass of a proton or neutron, so electrons contribute virtually nothing to the total mass of an atom. Electrons have an electric charge of (-1), which is equal but opposite to the charge of a proton, which is (+1). All atoms have the same number of electrons as protons, so the positive and negative charges 'cancel out', making atoms electrically neutral.
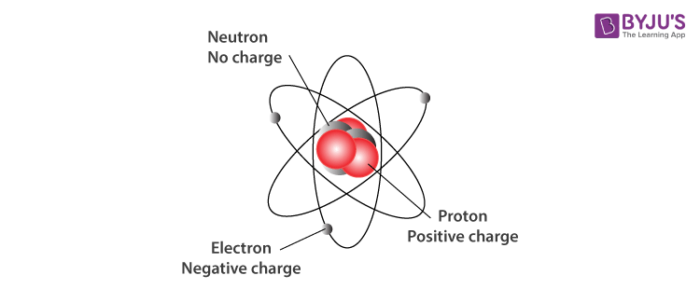
Unlike protons and neutrons, which are located inside the nucleus at the center of the atom, electrons are found outside the nucleus. Because opposite electric charges attract one another, negative electrons are attracted to the positive nucleus. This force of attraction keeps electrons constantly moving through the otherwise empty space around the nucleus. The figure below is a common way to represent the structure of an atom. It shows the electron as a particle orbiting the nucleus, similar to the way that planets orbit the sun. However, this is an incorrect perspective, as quantum mechanics demonstrates that electrons are more complicated.
Protons
A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (left( +1 right)) and a mass of 1 atomic mass unit (left( text{amu} right)), which is about (1.67 times 10^{-27}) kilograms. Together with neutrons, they make up virtually all of the mass of an atom.
Neutrons
Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Unlike protons and electrons, which are electrically charged, neutrons have no charge—they are electrically neutral. That's why the neutrons in the diagram above are labeled (n^0). The zero stands for 'zero charge'. The mass of a neutron is slightly greater than the mass of a proton, which is 1 atomic mass unit (left( text{amu} right)). (An atomic mass unit equals about (1.67 times 10^{-27}) kilograms.) A neutron also has about the same diameter as a proton, or (1.7 times 10^{-15}) meters.
What Is Subatomic Particles
As you might have already guessed from its name, the neutron is neutral. In other words, it has no charge whatsoever and is therefore neither attracted to nor repelled from other objects. Neutrons are in every atom (with one exception), and they are bound together with other neutrons and protons in the atomic nucleus.
Before we move on, we must discuss how the different types of subatomic particles interact with each other. When it comes to neutrons, the answer is obvious. Since neutrons are neither attracted to nor repelled from objects, they don't really interact with protons or electrons (beyond being bound into the nucleus with the protons).
Atom Subatomic Particles
Even though electrons, protons, and neutrons are all types of subatomic particles, they are not all the same size. When you compare the masses of electrons, protons, and neutrons, what you find is that electrons have an extremely small mass, compared to either protons or neutrons. On the other hand, the masses of protons and neutrons are fairly similar, although technically, the mass of a neutron is slightly larger than the mass of a proton. Because protons and neutrons are so much more massive than electrons, almost all of the mass of any atom comes from the nucleus, which contains all of the neutrons and protons.

| Particle | Symbol | Mass (amu) | Relative Mass (proton = 1) | Relative Charge | Location |
|---|---|---|---|---|---|
| proton | p+ | 1 | 1 | +1 | inside the nucleus |
| electron | e− | 5.45 × 10−4 | 0.00055 | −1 | outside the nucleus |
| neutron | n0 | 1 | 1 | 0 | inside the nucleus |
Table (PageIndex{1}) gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in 'atomic mass units.' An atomic mass unit ((text{amu})) is defined as one-twelfth of the mass of a carbon-12 atom. Atomic mass units ((text{amu})) are useful, because, as you can see, the mass of a proton and the mass of a neutron are almost exactly (1) in this unit system.
Negative and positive charges of equal magnitude cancel each other out. This means that the negative charge on an electron perfectly balances the positive charge on the proton. In other words, a neutral atom must have exactly one electron for every proton. If a neutral atom has 1 proton, it must have 1 electron. If a neutral atom has 2 protons, it must have 2 electrons. If a neutral atom has 10 protons, it must have 10 electrons. You get the idea. In order to be neutral, an atom must have the same number of electrons and protons.
Summary
Positively Charged Subatomic Particles
- Electrons are a type of subatomic particle with a negative charge.
- Protons are a type of subatomic particle with a positive charge. Protons are bound together in an atom's nucleus as a result of the strong nuclear force.
- Neutrons are a type of subatomic particle with no charge (they are neutral). Like protons, neutrons are bound into the atom's nucleus as a result of the strong nuclear force.
- Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive as an electron).
- The positive charge on a proton is equal in magnitude to the negative charge on an electron. As a result, a neutral atom must have an equal number of protons and electrons.
- The atomic mass unit (amu) is a unit of mass equal to one-twelfth the mass of a carbon-12 atom
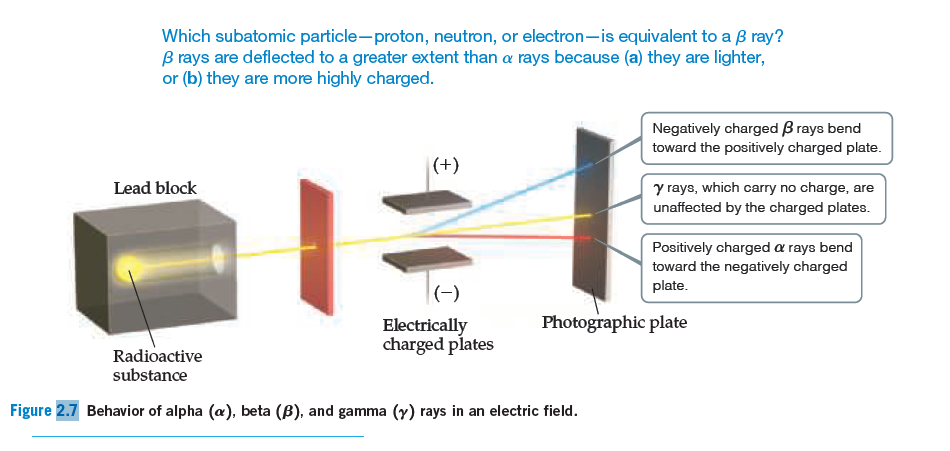
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Subatomic Particles Definition
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)

